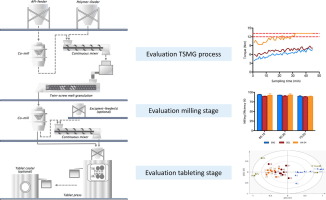
The concept of twin-screw melt granulation (TSMG) has steadily (re)-gained interest in pharmaceutical formulation development as an intermediate step during tablet manufacturing. However, to be considered as a viable processing option for solid oral dosage forms there is a need to understand all critical sources of variability which could affect this granulation technique. The purpose of this study was to provide an in-depth analysis of the continuous TSMG process in order to expose the critical process parameters (CPP) and elucidate the impact of process and formulation parameters on the critical quality attributes (CQA) of granules and tablets during continuous TSMG. A first part of the study dealt with the screening of various amorphous polymers as binder for producing high-dosed melt granules of two model drug (i.e. acetaminophen and hydrochlorothiazide). The second part of this study described a quality-by-design (QbD) approach for melt granulation of hydrochlorothiazide in order to thoroughly evaluate TSMG, milling and tableting stage of the continuous TSMG line. Using amorphous polymeric binders resulted in melt granules with high milling efficiency due to their brittle behaviour without producing excessive amounts of fines, providing high granule yields with low friability. Therefore, it makes them extremely suitable for further downstream processing. One of the most important CPP during TSMG with polymeric binders was the granulation-torque, which - in case of polymers with high Tg - increased during longer granulation runs to critical levels endangering the continuous process flow. However, by optimizing both screw speed and throughput or changing to polymeric binders with lower Tg it was possible to significantly reduce this risk. This research paper highlighted that TSMG must be considered as a viable option during formulation development of solid oral dosage forms based on the robustness of the CQA of both melt granules and tablets.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact