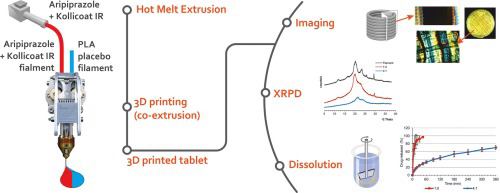
Three-dimensional printing is one of the fastest developing technology withinpharmaceutical field. With many advantages this method can be found as a new dosageform manufacturing technique, however low printing efficiency stays as one of the major limitations. Therefore, the preparation of filaments as a feedstock and printing of the final dosageforms in pharmacies may by the direction of development for this method. Thus, simple dosage and dissolution profile modification seems to be essential. This can be done in simple way by additiondrug-free filament during printing process. In this work the influence of dual co-extrusion process on the properties of 3D-printed tablets with aripiprazole was evaluated. A ZMorph® 3D printerequipped with DualPro extruder was employed to produce tablets made from Kollicoat® IR aripiprazole-loaded filament and commercially available PLA filament used to modify the release profile. Opticaland polarized light microscopy were utilized to evaluate structure of printed objects and X-ray diffraction studies were performed to determine crystallinity of aripiprazole withinfilament and tablets. Fast dissolution of aripiprazole resulted from its amorphization while prolonged drug release was a result of co-extrusion with PLAfilament. Importantly, the drug remained crystalline within the filament and phase transition into disordered system appeared during printing of tablets. Given the high stabilityof crystalline materials such feature is especiallybeneficial for long-term storage of feedstock filament.