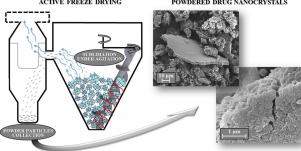
Abstract
Active Freeze Drying allows for producing lyophilised powders by progressive agitation of frozen blocks undergoing sublimation. One potential application of this process is the formulation design of unstable nanosuspensions for oral drug delivery, as here shown for nanocrystal-based ketoconazole powder. With this technique, a critical vapour flow needs to be achieved in order to obtain reasonable process yields (>78%). The size distribution of powder particles (median size between 21 and 44 µm) was affected by the nanocrystals concentration and the drug-to-stabilizer ratio. This was assumed to be related to the mechanical strength of the solid network from which the powder particles break off. The adjustments of the drug-to-stabilizer ratio and the freezing procedure proved to play a major role in improving powder redispersibility. However, differences in powder redispersibility did not translate into significant changes in in-vitro dissolution rates. Active Freeze Drying has confirmed to be a promising tool to efficiently produce redispersible nanocrystal powders.
Conclusions
Among the process parameters examined in the present study, the jacket temperature was the main factor affecting the process performance (i.e. the average rate of vapor transfer and the yield). In particular, the results revealed that the vast majority of the fragments breaking off the dry layer are transported out of the chamber when the vapour flow reaches a critical threshold corresponding to a critical jacket temperature. This suggests that a product does not need to be dried under highly aggressive conditions (i.e. above this critical jacket temperature) to get high collector yields.
Apart from the effect of the vapour flow, the size distribution of the powder fragments collected was influenced by the nanocrystal concentration and the TPGS-to-drug ratio, as these factors presumably modify the structure of the nanocrystal network from which these fragments break off. This provides a first insight on how the size of the powders particles could be adjusted to some extent by controlling the mechanical strength of the original network. However, the cohesive behaviour of the fine powder particles generated is not considered as a limiting factor for now to be processed into final solid dosage forms (e.g. hard capsule filling or tableting). Indeed, additional formulation (e.g. adjunction of glidant) and/or processing steps (e.g. granulation on line) could be implemented to improve their flowability.
Finally, the TPGS-to-drug ratio and the freezing procedure proved to play a major role in improving powder redispersability. Minimization of aggregation did not however result in faster dissolution of ketoconazole nanocrystals. Redispersion cannot be therefore considered and a rate-controlling step of the dissolution process in this case.
Altogether this study showed that AFD is a promising technology for the production of nanocrystal-based powders.