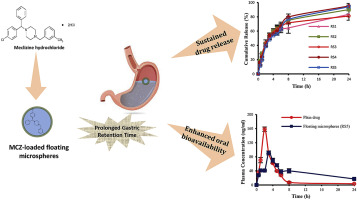
The aim of this study was to investigate whether floating microspheres prepared from polymer blends of hydroxypropyl methylcellulose in combination with either Eudragit S100 or Eudragit RS100 could
improve the systemic bioavailability of the antiemetic drug; meclizine HCl (MCZ). Floating microspheres containing MCZ were prepared by emulsion solvent evaporation method. The influence of different
polymer blend compositions on various formulation parameters and in
vitro release characteristics of the prepared microspheres was investigated. In addition, the in
vivopharmacokinetic profile of an optimized formula was evaluated. The mean particle size of prepared microspheres ranged from 290 to 383 μm. The percent drug entrapment efficiency varied
between 51.9 ± 0.3% to 91.6 ± 0.7%. The formulated microspheres exhibited excellent floating ability. In
vitro release profiles of floating microspheres indicated a rapid initial release followed by a sustained drug release for up to 24 h. The pharmacokinetic data affirmed the sustained
effect of the optimized formula as indicated by the prolonged tmax, compared to the plain drug. Furthermore, the optimized
formula showed higher relative bioavailability (154.25%), compared to the plain drug. These results suggest that MCZ floating microspheres might represent potential alternative to conventional MCZ
oral formulations, with a higher and sustained therapeutic efficacy.