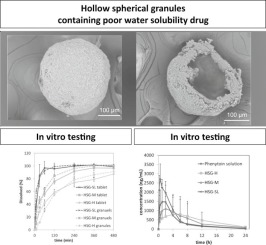
Abstract
Water-soluble polymers with high viscosity are frequently used in the design of sustained-release formulations of poorly water-soluble drugs to enable complete release of the drug in the gastrointestinal tract. Tablets containing matrix granules with a water-soluble polymer are preferred because tablets are easier to handle and the multiple drug-release units of the matrix granules decreases the influences of the physiological environment on the drug. However, matrix granules with a particle size of over 800 μm sometimes cause a content uniformity problem in the tableting process because of the large particle size. An effective method of manufacturing controlled-release matrix granules with a smaller particle size is desired. The aim of this study was to develop tablets containing matrix granules with a smaller size and good controlled-release properties, using phenytoin as a model poorly water-soluble drug. We adapted the recently developed hollow spherical granule granulation technology, using water-soluble polymers with different viscosities. The prepared granules had an average particle size of 300 μm and sharp particle size distribution (relative width: 0.52–0.64). The values for the particle strength of the granules were 1.86–1.97 N/mm2, and the dissolution profiles of the granules were not affected by the tableting process. The dissolution profiles and the blood concentration levels of drug released from the granules depended on the viscosity of the polymer contained in the granules. We succeeded in developing the desired controlled-release granules, and this study should be valuable in the development of sustained-release formulations of poorly water-soluble drugs.
Conclusion
We attempted to develop matrix-type, sustained-release granules for the poorly water-soluble drug, phenytoin. The new granulation technology OPUSGRAN® was adapted to manufacture ideal granules for sustained-release tablets in a simple manner with a short manufacturing time. A water-soluble, high-viscosity polymer was used to control the drug dissolution rate and enable drug release from the granules in the lower gastrointestinal tract. We successfully manufactured the HSGs using a fluidized bed rotor granulator. The granules showed sustained-release dissolution profiles in dissolution testing in vitro. The dissolution release profiles depended on the viscosity of the constituent water-soluble polymer. The dissolution profiles of the HSGs did not change after compression, and this shows that the HSGs can be adapted for tablet manufacture. The sustained-release effect in vivo was evaluated in rats. The blood concentration profiles of phenytoin released from the granules depended on the viscosity of the constituent water-soluble polymer, and good bioavailability was maintained. As mentioned above, we have successfully prepared controlled-release matrix granules with a high drug loading and the granules can also be used in tablets. This study indicates a new strategy to prepare high drug loading sustained-release formulations for small-sized tablet.