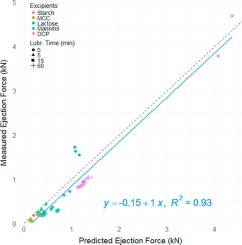
Pharmaceutical powders can exhibit markedly different tablet ejection forces. The purpose of this study is to understand the factors leading to the variability of the tablet ejection force and its sensitivity to lubrication. The study showed that the tablet ejection force is mainly governed by 1) tablet diameter and thickness, 2) compact-die wall friction coefficient, and 3) residual die wall stress upon ejection; the latter was further controlled by the maximum compression pressure, as well as the degree of non-elastic deformation during compression. Brittle powders, such as lactose or dicalcium phosphate, often exhibit exceeding ejection force because of their significant contribution from the non-elastic deformation during loading. These conclusions were verified through compaction experiments of five pharmaceutical powders with diverse compaction properties. Additionally, we found that boundary lubrication was highly effective in reducing tablet ejection force, achievable through decreasing the compact-die wall friction coefficient, but not through altering the intrinsic consolidation behavior of powders. High ejection force is indicative of the sub-optimal stress condition of the tablet post-unloading. Therefore learnings from this study are beneficial for practitioners to harness the ejection force as an effective metric to identify and mitigate risks of tablet defects.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact